|
|||
|
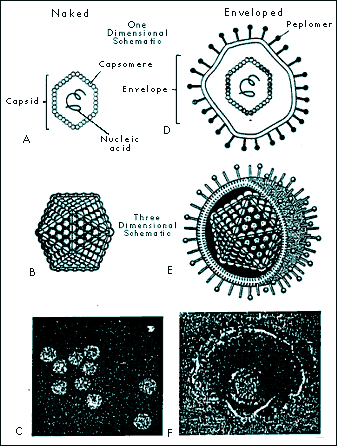
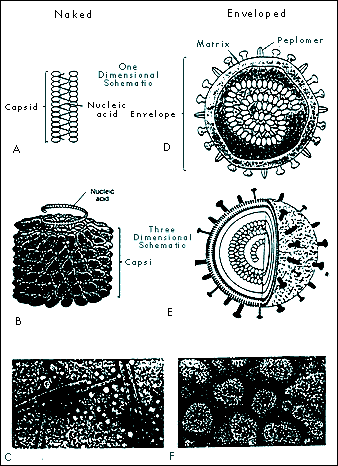
A Virology Primer: With Special Reference to Ozone Gerard Sunnen, M.D. The Nature of Viruses Components of Viruses 2. The nucleic acid coat or capsid. Surrounding the core is a protective coating made of protein, the capsid. The capsid is rigid and determines the shape of the virus. It is made of repeating protein units called capsomeres. The architecture of capsomere assembly is quite fascinating. A common crystal-like configuration is the 20-sided isocahedral construction, with each capsomere forming an equilateral triangle. Another pattern is helical or rod shape. The nucleic material and its capsid curl unto itself forming a spherical structure. A few viruses have capsids that are neither icosahedral nor helical. These configurations are termed complex, and may be spiral, brick shaped, or otherwise non-standard in their appearance. The capsid and its nucleic acid core together form the nucleocapsid.
5. Virions are the mature viral particle capable of infecting other cells. In non-enveloped viruses, the virion consists of the nucleocapsid alone. For enveloped viruses, the nucleocapsid and the envelope make up the virion. Enveloped viruses are only comfortable in the delicate homeostatic environment of mammalian bodily fluid milieus and are transmitted by bodily fluid transfers such as blood transfusion and sexual contact. The intestinal route, on the other hand, usually transmits naked viruses. Enveloped Viruses Include the Following Families: Some Major Non-Enveloped (Naked) Viruses: Novel infectious agents Satellites are tiny RNA fragments dependent upon the enzymes of viruses for securing their progeny. Viroids, closed circular RNA molecules without capsids, do not require the presence of a helper virus. These botanical infectious agents, however, need the RNA polymerases of the cells they invade. Prions, in humans, are responsible for Creutzfeldt-Jakob disease, familial fatal insomnia, Gerstmannaussler-Scheinker disease, and kuru. These diseases inexorably ravage the nervous system. In animals, they cause scrapie in sheep, chronic wasting disease in elk, and bovine spongiform encephalopathy, among others. Prions are transmissible infectious particles composed of quasi-facsimiles of normal body proteins. They differ, however, in that they contain only a few amino acid substitutions sufficiently minor as to make them invisible to the body’s immune surveillance. Commentaries on the Evolution of Viruses In the 21st century, this ecology, namely the human reservoir, has changed dramatically. The eruptive world population and the mobility of the planet's inhabitants are two major factors responsible for the accelerated evolution of viruses into new frontiers of pathogenicity. Most of the families of viruses mentioned above have produced new pathogenic strains. Of great concern is the diversification of the retroviral, the hepatitis and the influenza families. In the past three decades, for example, the following viruses are among the many that have been discovered: human herpesvirus 6, 7, and 8; hepatitis C; hepatitis E; morbillivirus (encephalitis); and hantaviruses. Viral Infection Research has shown that the quantity of virions involved in infection is much more important than previously realized. In hepatitis C, retroviruses, and influenza, for example, several billion new particles may be manufactured daily. Viral load is a measure of the virion population. Understandably, the immune system is placed under sustained pressure to neutralize these waves of new infective arrivals and to regenerate its own decimated cellular components. Any reduction in the viral load by any means proffers an advantage to the immune system and thus enhances the probability for vanquishing the infection. Ozone, a natural molecule The oxygen atom exists in nature in several forms: (1) As a free atomic particle (0), it is highly reactive and unstable. (2) Oxygen (02), its most common and stable form, is colorless as a gas and pale blue as a liquid. (3) Ozone (03), has a molecular weight of 48, a density one and a half times that of oxygen, and contains a large excess of energy in its molecule (03 ⇒3/2 02 + 143 KJ/mole). It has a bond angle of 127° ± 3°, resonates among several hybrid forms, is distinctly blue as a gas, and dark blue as a solid. Ozone is a powerful oxidant, surpassed in this regard only by fluorine (4) 04 is a very unstable, rare, nonmagnetic pale blue gas that readily breaks down into two molecules of oxygen. Ozone application for medicinal purposes is a specialized discipline requiring knowledge of ozone’s physical, physiological and antimicrobial dynamics.The ozone inactivation of viruses Viruses. Viruses are parasites at the genetic level, separated into families based on their structures, types of nucleic genome and modes of replication. Recently, there has been renewed interest in the potential of ozone for viral inactivation in vivo. It has long been established that ozone neutralizes viruses in aqueous media and it stands to reason that it would be studied for similar applications in living systems. In vivo ozone applications, however, present far greater challenges. Indeed, the technology of medical ozone administration aims to respect the delicate balance of patient safety on one hand and antimicrobial efficacy on the other. All viruses are susceptible to ozone's neutralizing action. Viruses, however, differ in their relative susceptibility to destruction by ozone. In one study, poliovirus resistance was 40 times that of coxsackievirus. Relative susceptibility in ascending order was found to be: poliovirus type 2, echovirus type 1, poliovirus type 1, coxsackievirus type B5, echovirus type 5, and coxsackievirus type A9. In pure water, at maximal solubility of ozone and room temperature, echovirus type 29 is inactivated in one minute, poliovirus type 1 in two, type 3 in three, and type 2 in seven minutes (Roy 1982). Analysis of viral components showed damage to polypeptide chains and envelope proteins, which could result in attachment capability compromise, and breakage of the singleanded RNA producing replicating dysfunction. Other researchers, in similar experiments, concluded that in ozonation, it is the viral capsid that sustains damage (Riesser 1977). Viruses, unlike mammalian cells, have no enzymatic protection against oxidative stress. Lipid-enveloped viruses are sensitive to treatment with ether, organic solvents, and ozone (Bolton 1982), indicating that disruption or loss of lipids results in impaired or destroyed infectivity. The enveloped viruses are more fragile than the naked viruses. Envelopes, made up of lipids, phospholipis, cholesterols and glycoproteins are especially vulnerable to ozone's capacity for electron removal (oxidation). Cleaving the unsaturated chemical bonds of envelope peplomers damages the capacity of viruses for attaching to host cells and progressing in their life cycles. In addition, once the virion’s lipid envelope becomes fragmented, its genomic core cannot survive. The enveloped viruses, adapted to the delicate homeostatic milieu of their mammalian hosts are usually more sensitive to all physico-chemical challenges than are naked virions. Although ozone's effects upon unsaturated complex lipids are one of its best documented biochemical action, ozone is known to interact with other viral constituents. This becomes relevant when ozone inactivation of non-enveloped virions is considered. In contrast to enveloped viruses, naked viruses constituted of a nucleic acid core and a protein capsid coating, usually show more robust resistance to chemical and oxidative challenges. This is logical, in view of their dependence upon survival in harsh environmental habitats, such as contaminated waters and fomites. Ozone, however, has the capacity to inactivate all naked viruses. Indeed, when ozone comes in contact with viral capsid proteins, protein hydroxides and protein hydroperoxides are formed, and viral demise ensues. In summary, ozone's antiviral action in blood may recruit the following mechanisms: 1. The denaturation of virions through direct contact with ozone. Ozone, via this mechanism, disrupts viral envelope lipids, phospholipids and lipoproteins. The presence of numerous chemical double bonds in these molecules makes them vulnerable to the oxidizing effects of ozone, which readily donates its oxygen atom and siphons electrons in redox reactions. Broken bonds are thus reconfigured, molecular architecture becomes disrupted, and breakage of the viral envelope ensues. Deprived of an envelope, virions cannot sustain nor replicate themselves. 2. Ozone proper may directly alter structures on the viral envelope that are necessary for attachment to host cells. Peplomers, the viral glycoproteins protuberances that connect to host cell receptors are likely sites of ozone action. Alteration in peplomer integrity impairs attachment to host cellular membranes foiling viral attachment and penetration. 3. Introduction of ozone into the serum portion of whole blood induces the formation of lipid and protein peroxides. While these peroxides are not toxic to the host in quantities produced by ozone therapy, they nevertheless possess oxidizing properties of their own which persist in the bloodstream for several hours. Peroxides created by ozone administration may serve to further reduce viral load by sustained anti-peplomer action. 4. Immunological effects of ozone have been documented. Cytokines are proteins manufactured by several different types of cells that regulate the functions of other cells. Mostly released by leucocytes, they are important in mobilizing immune response. Ozone induces the release of cytokines that in turn activate a spectrum of immune cells. Ozone is reported to be an immuno-stimulant in low doses and immuno-inhibitory at higher levels (Werkmeister 1985, Varro 1974, Zabel 1960). Additionally, ozone functions as a signaling agent by stimulating production of nuclear factor kappa B, interleukin 6, and tumor necrosis factor æ. Ozone’s capacity for cytokine activation has been amply documented (Bocci 2005). 5. Ozone action on viral particles in infected blood yield several possible outcomes. One outcome is the modification of virions so that they remain structurally intact yet sufficiently dysfunctional as to be nonpathogenic. This attenuation of viral particle functionality through slight modifications of the viral envelope, and possibly the viral genome itself, mollifies pathogenicity and allows the host to increase the sophistication of its immune response. The creation of dysfunctional viruses by ozone offers unique therapeutic possibilities. In view of the fact that so many mutational variants exist in any one afflicted individual (e.g., hepatitis C, influenza, HIV), the creation of an antigenic spectrum of crippled and fragmented virions could provide for a unique host-specific stimulation of the immune system, thus designing what may be called a host-specific autovaccine. 6. An exciting avenue of research suggests that the virucidal properties of antibodies are predicated upon their ability to catalyse highly active forms of oxygen including ozone (Marx 2002; Wentworth 2002). In this model, activated neutrophils provided with appropriate starting materials are capable of generating singlet oxygen, a most powerful oxidant. The singlet oxygen combines with oxygen to form ozone, itself an oxidant, whose electron-extracting capacity is only second to fluorine. It can also combine with water to form the hydroxyl radical (OH) and hydrogen peroxide. Endogenously created ozone thus becomes a fundamental immunological agent for viral inactivation. Exogenously administered ozone may, based on this model, amplify the efficacy of antigen-antibody dynamics. Methodologies of ozone antiviral therapy Importantly, another, more experimental and more intensive technique of ozone administration makes use of the extracorporeal treatment of the entire blood volume using a hollow-fibre oxygenator-ozonizer (Di Paolo 2000; Bocci 2002). This approach is promising because all blood and lymphatic fluids are interfaced with oxygen/ozone gaseous mixtures thus providing direct integral anti-viral therapy. Research is needed to determine the clinical indications and the appropriate protocols for this promising ozone/blood interfacing methodology. Ozone is an exemplary agent for neutralizing a wide range of viruses. Medical ozone technology spares the biological integrity of host tissues, including blood. As regards the viability of cellular elements such as red blood cells, leucocytes and platelets, the oxidizing potential of ozone may be accurately calibrated so that it will be fatal to virions but innocuous to host cells. Ozone has a propitious therapeutic range of administration. Like many other drugs, ozone may be said to optimally function within a therapeutic window defining its optimal levels of administration. Below the window, concentrations and dosages of administered ozone are poorly effective; within the window they function optimally; and above the window, toxic effects occur. Ozone offers distinct features over other antiviral agents. It is above all, a gas. When a gas is administered to a liquid an immediate dispersion of the gas takes place thus effecting reactions in the entire fluid volume. This may be contrasted to a fluid additive, such as hydrogen peroxide, that commixes much more sluggishly with the biological fluid under treatment due to the physics of fluid-to-fluid dynamics. What is more, ozone, unlike drug therapies, is impervious to viral mutational defenses. Viremic episodes represent sudden invasions of virions into bodily fluids. In the case of acute infections such as avian influenza, there may be one major viremic episode which, depending upon host factors, may be marked by high lethality. Interventions that will safely decrease the numbers of virions from the circulation will proffer an advantage to beleaguered immune functions. Viral culling via ozone hemotherapy consists in the serial treatment of aliquots of blood with precise doses of ozone. Extracorporeal ozone therapy treats the entire blood and lymphatic circulations. Judiciously decreasing viral load during viremic episodes allows for the recovery of besieged immunocompetence. Conclusion, and Commentaries on Ozone Research Of all the antiviral agents known, none offer the special advantages ozone embodies. Ozone, because of its potent oxidizing power is able to deactivate all known viruses. It is a pan-virucidal agent, whose mechanisms of action viruses are, at this time, unable to counter. Technologies with impeccable safety profiles have been developed to deliver ozone/oxygen mixtures to virally-afflicted patients. As a gas, ozone is incomparably able to penetrate fluids with ease and rapidity. Ozone has both immediate antiviral physico-chemical and longer-term immunological effects. Research is compellingly needed to understand the deeper mechanisms of ozone formation in the immune system so that novel antipathogenic therapies may be discovered for responding to increasingly urgent public health needs. Research is also needed to determine the indications for ozone administration relative to specific viral infections and to delineate disease-specific treatment protocols. Promising antiviral technologies include serial ozone hemotherapy and total blood and lymphatic system extracorporeal ozonation. Viral diseases are expanding worldwide. This is not a science fiction scenario but a regrettable fact. Old diseases are not only finding increased population reservoirs but are also developing novel mechanisms of infectivity. Of special concern are emergent viruses, products of mutational creativity, never before encountered by humans, that are potentially devastating to health and life. Ozone embodies unparalleled promise for becoming a major contributor to the armamentarium against these new plagues. References Ackey D, Walton TE. Liquid-phase study of ozone inactivation of Venezuelan Equine Encephalomyelitis virus. Appl Environ Microbiol 1985; 50:882-886
BACK TO MENU |